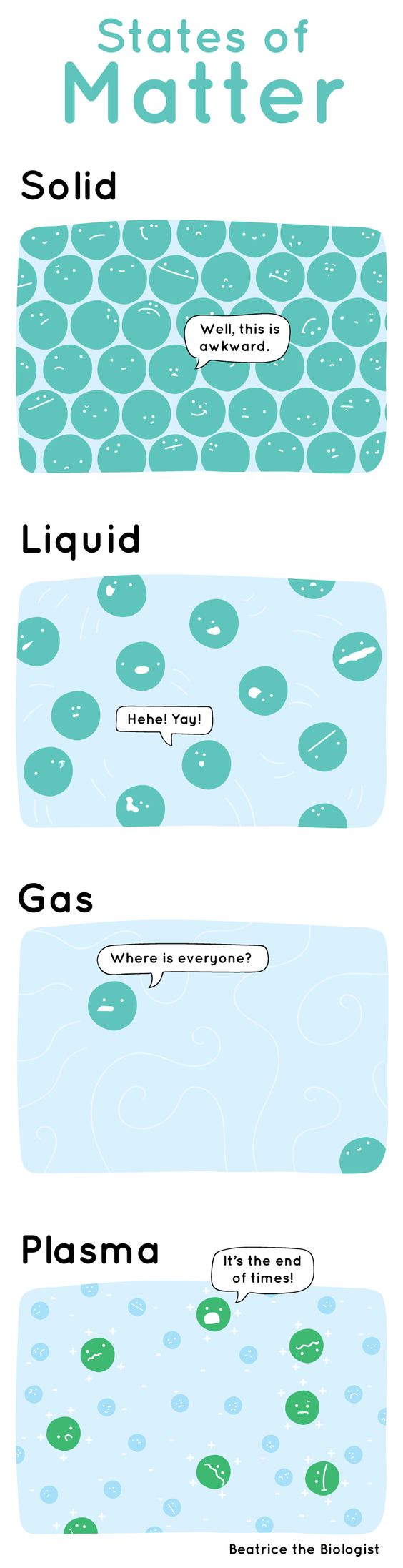
Intermolecular Forces
|
Essential Question:
Why does sugar dissolve faster in hot water? Learning Objective: I can construct an explanation for how temperature affects the rate of reaction. Standards addressed: HS-PS1-5. Apply scientific principles and evidence to provide an explanation about the effects of changing the temperature or concentration of the reacting particles on the rate at which a reaction occurs. HS-PS1-6. Refine the design of a chemical system by specifying a change in conditions that would produce increased amounts of products at equilibrium.* |